Why the Solution to PTFE Adhesion to Guidewires is Not a Material Swap
As seen in Q-Med
The demand by the EPA that manufacturers eliminate PFOA from their PTFE formulations by 2015 appears related to a series of crises for some medical manufacturers due to loss of coating adhesion on guidewires. A recent FDA Class I recall notice states: “There is a potential for PTFE (polytetrafluoroethylene) coating to delaminate and detach from guide wires.” The FDA goes on to say, “Uses of this recalled product may result in serious adverse health consequences.”

What We Know
The delamination problem appeared coincidental with the reduction and eventual elimination of PFOA. PFOA was used as a surfactant in water-based PTFE formulations. It was thought to enhance adhesion, particularly on smooth wire products that were marginally pre-cleaned. Empirical evidence supports this conclusion, as flaking and delamination were unheard of with the earlier formulations that contained PFOA. However, saline testing appeared in 2012, there were reports of “green flakes” in an operating room when PTFE coated guidewires were immersed in a saline soak tank prior to an intravascular procedure. Now medical device manufacturers wanted a saline soak test performed at the coating facility. The PTFE coating process, in the past, may have been less sensitive to saline, but not anymore.

Thin PTFE films are porous. Saline, in concentrations that are common in medical procedures, does permeate the film. The saline reacts with the surface interface and if not perfectly adhered, the PTFE bond will fail (Figure 1). For some coating applicators, the loss of PFOA was related to “sudden” erratic adhesion. Dry tape testing of the packaged and coated guidewires showed no delamination. But the same coated wires, soaked in saline, failed 100%. Eventually, this resulted in FDA Class 1 recalls of guidewires. To assure adhesion, PTFE-coated devices can be saline soaked and 100% tape tested. Providing the coating passes the saline soak and tape test, the device can be packaged, sterilized and used.
The coating applicator had used water based, “pure” PTFE coatings for years. The FDA recalls called for immediate action. The coating manufacturer was involved in the investigation of the recall. They explained that this appeared to be an isolated incident. During this investigation, the engineers from the device manufacturer learned that the coating manufacturer, in addition to the pure PTFE coating, manufactured resin-bonded coatings that do contain PTFE. Now, all coatings sold in the US comply with the no-PFOA, EPA mandate. So, some device engineer’s reflex reaction was to now specify the resin-bonded PTFE coatings that have better adhesion to most surfaces. The driving force was to retrieve the adhesion that was lost. That may be a very shortsighted remedy.
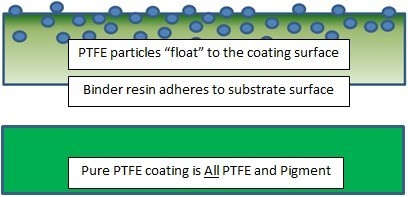
Many coating manufacturers also make these coatings that contain PTFE, but that is where the similarity ends.
Based on the particular PTFE that is dispersed in engineering resins (such as PAI and PES), these resin-bonded coatings have a long, successful history as barrier coatings and as bearing surfaces on products ranging from saw blades to throttle shafts for automobiles. They are tough, tenacious, and abrasion resistant. However, these resin-bonded coatings have neither the extremely low friction nor flexibility pure PTFE coatings.
The resin-bonded coatings cannot achieve the low coefficient of friction commonly found in pure, water-based PTFE coatings that have been historically specified for five decades on guidewires and other medical devices. The coefficient of friction for pure PTFE is approximately 0.02-0.06; the resin-bonded based coatings, on the other hand-even those that are said to have the PTFE particles “stratified” on the coating surface-have coefficients of friction in the range of 0.10 to 0.16. This is because much less of the PTFE is exposed at the surface of the resin bonded formulations.
The potential change to resin-bonded coatings results in two related problems. One is tactile or ergonomic; the other is mechanical.
The higher friction of the resin-bonded films results in greater drag when inserted in vascular systems. Thus, the “touch” felt by the attending physician is not the same as he/she is used to, and is considerably heavier.
Because the coefficient of friction with resin-bonded coatings is much higher, the guidewire probes encounter a phenomenon known as “stick-slip.” This occurs when a coated product momentarily stalls in its travel because the friction is greater than the driving force; then as force builds up, the probe lurches forward. Pure PTFE coatings do not experience this phenomenon, but instead transition from a state of static friction to kinetic friction smoothly.
To the operating physician, guidewires with resin-bonded coatings feel “heavier” and move with a hesitating motion. This hesitation can be perceived as an unknown obstruction. Guidewires coated with pure PTFE, on the other hand, produce a consistently light feel as they travel through the bends of a vascular system. Thus when an obstruction is reached, the operating physician instantly knows that a problem has been encountered.
A Problem Turning Corners
Another limitation of resin-bonded coatings is limited elongation. They are capable of about 40% elongation, and then tend to crack or delaminate from a substrate at elongations greater than that. This is due to the inherent mechanical properties of the engineering resin binders.
Some of the turns within a vascular system are sufficient to exceed this limit from the inside of the turn to the outside on a guidewire. The resin-bonded coating can be stressed to the point that it cracks or even flakes off. These materials cannot satisfy the requirements for the International Organization for Standardization test for coated guidewires (ISO 11070:1998E, sections 8.4 and 8.5). Also, they commonly fail the ISO mandrel test (ISO 7802:2013E) in which guidewires coated are turned around a mandrel. Because the resin-bonded systems cannot stretch sufficiently, they crack.
Pure PTFE coatings, on the other hand, have at least double the elongation of resin-bonded coatings and have no problem passing these critical tests. Simply, all coatings labeled “PTFE” are not the same as coatings that contain PTFE.
Is the Future the Past?
For over five decades, pure PTFE coatings were the mainstay for guidewires and related products. What made them ideal for medical products earlier-the unmatched low friction coefficient-was the reason pure PTFE coatings became the “gold standard” for medical device coatings until the reformulation to remove PFOA resulted in adhesion problems and recalls.
But these problems did not need to happen because there is a way to apply reformulated PTFE to guidewires with no loss of adhesion or delamination. Surface Solutions Group (SSG) of Chicago discovered that by using new stringent surface preparation techniques and adjusting how the coating is applied makes the problem go away and the new coatings adhere as well as the old ones did.
Such a breakthrough is not unusual at SSG. Applying low-friction films to biomedical products-from stainless steel, titanium, Nitinol, and even plastic or glass-has been their exclusive focus. For the guidewire problem, they are able to bond pure PTFE-containing no PFOA-so tenaciously that delamination is eliminated, even after saline exposure, tape test, mandrel wrap test, and cross hatch/ boil and fingernail tests.
This is how the process works: The wire surface is hyper cleaned, and then the coating is applied in a controlled environment, using a robotic system to keep precise control of all the physical parameters (pressure, feed rate, and electrostatic level) in the spray system so that only an exact amount of coating is deposited to the wire. Finally, every individual part can be saline and tape tested, per the OEM’s requirements if necessary.
Both patient and physician benefit from the SSG breakthrough. The patient benefits from the improved safety of guidewires that won’t delaminate. The physician benefits from guidewires that, once again, deliver the smooth tactile “feel” that he counted on from wires that were coated with the original gold standard coating.
Click Here to view or download this article in PDF form
